Abstract
Background:
Venous thromboembolism (VTE) incidence in hematological cancer patients greatly influences their morbidity and mortality. However, VTE complications in acute leukemia (AL) received little attention in comparison to other more frequently occurring complications, such as thrombocytopenia-associated bleeding and granulocytopenia related severe infection.
Methods:
We conducted a systematic review to systematically assess the incidence rate of VTE in the setting of AL, including acute myeloid leukemia (AML), acute promyelocytic leukemia (APL), and acute lymphoid leukemia (ALL), using the information from available observational studies and randomized trials. A meta-analysis of proportions using fixed and random effects models was performed. Herein, we report all the outcomes in random model estimates.
Results:
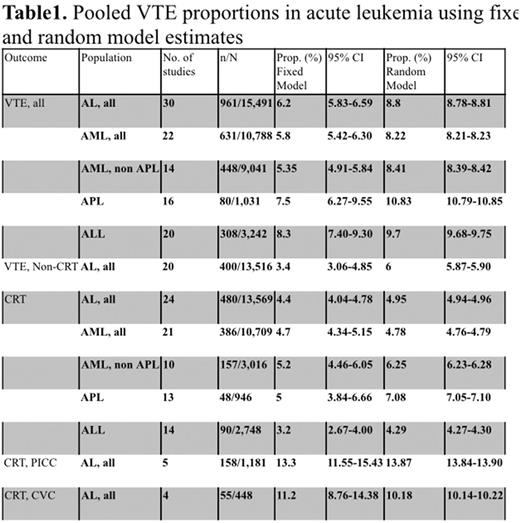
Thirty studies were included (29 observational and 1 case control) with a total of 15,491 AL participants in (age range: 15-91). Of the total participants, the number of evaluable patients was 10,788 in AML, 3,242 in ALL, and 1,031 in APL. The VTE rate was found to be 8.8% (95%CI: 8.78-8.81) in all AL patients, 8.2% (95%CI: 8.21-8.23) in AML, 9.7% (95%CI: 9.68-9.75) in ALL, and 10.8% (95%CI: 10.79-10.85) in APL. The recurrence rate was 12% (95%CI: 9.03-15.08) in AL patients (n=435); 17% (95%CI: 11.02-24.26) with AML (n=135); 12% (95%CI: 7.61-18.33) with ALL (n=142) (AML vs. ALL: p=0.233). A subgroup analysis of catheter related thrombosis (CRT) demonstrated a rate of 4.95% in all AL participants (95%CI: 4.94-4.96), 4.7% (95%CI: 4.68-4.77) in AML, 4.3% (95%CI: 4.27-4.32) in ALL, and 7% (95%CI: 6.99-7.10) in APL. The rate of cerebral vein thrombosis in ALL patients treated with l-asparaginase was 2.8% (95%CI: 2.78-2.90).
Conclusion:
Our data demonstrates a significant incidence of venous thrombosis in AL patients, with a high recurrence rate among those patients. L-asparaginase seems to be particularly associated with a considerably high rate of cerebral thrombosis in ALL. However, there was a lack in randomized trials addressing thrombosis incidence in AL as a primary outcome. Given these significant incidence rates of thromboembolism, future studies are warranted to address the efficacy and safety of thromboprophylaxis in AL patients.
Lazo-Langner: Bayer: Honoraria; Daiichi Sankyo: Research Funding; Alexion: Research Funding; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.